Keck-phase-2
THIS DOCUMENT HAS BEEN MOVED TO WORD, CHANGES MADE HERE WILL NOT BE REFLECTED IN THE PROPOSAL.
Contents
- 1 1) Cover Page
- 2 2) Project Abstract
- 3 3) Project Narrative
- 3.1 1) Statement of the work to be undertaken and expected significance.
- 3.2 2) Objectives/goals for the proposed work.
- 3.3 3) Project timeline keyed to the objectives/goals.
- 3.4 4) Relation of the objectives to:
- 3.5 5) Concise description of methods and procedures for implementation and experimentation.
- 3.6 6) Technical problems that may be encountered and how they will be addressed.
- 3.7 7) Roles of all key project personnel.
- 3.8 8) Organization chart of key project personnel.
- 3.9 9) Description of facilities, equipment and resources available for the project.
- 3.10 10) Equipment requests should:
- 3.11 11) Plans for this project beyond the proposed time period, including financial support.
- 3.12 12) Describe how the success of the project will be evaluated in terms of the goals proposed. Include information regarding outside review committees, if appropriate.
- 4 4) Project Budget Form
- 5 5) Budget Narrative
- 5.1 1. Provide a brief justification of each budget line item.
- 5.2 2. State the number of students (undergrad/grad/postdoc), research associates or technicians to be supported and number of years of support.
- 5.3 3. Explain why W. M. Keck Foundation support is essential for this project.
- 5.4 4. List all current and pending federal and non-federal support, including institutional or departmental funding, related to this project.
- 5.5 5. If construction or remodeling is involved, provide a copy of the permits required or an explanation of how and when the permits will be acquired.
- 6 6) Recognition Statement
- 7 7) Project Documents
1) Cover Page
- Barbara's doc is here.
2) Project Abstract
- 150 word limit.
Earlham College requests $358,877 to develop multidisciplinary science curriculum modules emphasizing computational methods and student/faculty research projects both focusing on a common core problem: metals in the environment. This project will emphasize collaboration among our natural science departments, including biology, chemistry, computer science, geosciences, mathematics, and environmental science. Scientific research is becoming increasingly multidisciplinary, collaborative, and computational. Therefore, it is essential to train our students to develop multi-faceted approaches to problem solving that use both traditional laboratory techniques and computational methods. This project will introduce an important scientific problem (metals in the environment), ask students to collect and analyze data, and to make interpretations using different disciplinary perspectives. This idea of collaborative multidisciplinary learning will transform our undergraduate curriculum in the sciences and provide a model for programs among the sciences at other liberal arts colleges.
3) Project Narrative
- Maximum of 25 pages conforming to the following requirements:
- 8.5" by 11" paper
- Single spaced with all margins measuring at least 1"
- At least 12 point font in Times New Roman
- No more than 6 lines of type within a vertical space of 1"
- Proposals that do not comply with these requirements will not be accepted
- XXX Question: 25 pages for the project narrative section or the whole application?
- Define module - thematic unit with specific learning objectives
1) Statement of the work to be undertaken and expected significance.
This project will bridge the gap between modern scientific research and science education by incorporating research modules with a computational emphasis into courses and further developing multidisciplinary summer research activity. These research modules will be integrated into courses beginning with six introductory courses in chemistry, biology, geosciences, mathematics, and computer science and will become increasingly complex in nine upper level courses in these disciplines as well as environmental programs. Almost every one of Earlham's 1200 students will take at least one of these classes before they graduate. Both course modules and summer research projects will focus on a problem of local concern (the fate, transport and toxicity of heavy metals within a local watershed) and will include laboratory, field, and computational modeling components. Course modules augmented by summer research will contribute to the overall understanding of heavy metal biogeochemical cycling in the environment (e.g. development of new biotic ligand models). Additionally, our proposed research will contribute important location-specific information on heavy metals cycling that will benefit on-going municipal, state and federal pollution studies.
Students will actively participate in collecting, analyzing and interpreting data that will be integrated into ecological and human health risk assessments at the contaminated site. They will learn science by doing science in collaboration with a diverse array of peers, mentors and stakeholders. Students will have tangible "ownership." We anticipate that a renewed approach will invigorate student interest and participation in science - even in introductory courses. By reformulating our model of science education to greatly expand active student participation in meaningful research, we expect a strong impact on student learning. Such an impact will stimulate students to broaden their horizons in related coursework in different disciplines.
The outcome for students will be better retention of scientific information and a multifaceted literacy with the workings of modern science. This small effort will contribute to a more scientifically literate citizenry and, inevitably, will cultivate better scientists. For faculty, the outcome will be an enhanced perspective on the significance of one's own disciplinary contributions. Intentional demolition of disciplinary barriers will stimulate faculty to ask research questions that they can not presently imagine. The breadth of our proposed cross-pollination - almost the entire science division of the college - is unknown (and likely, impossible) at other institutions.
Four aspects of our project work together to make it powerful: 1) our focus on local problems; 2) the combined use of field, laboratory, and computational methods; 3) the longitudinal involvement of students as they take introductory through upper-level science classes; and 4) showing students how modern science is multidisciplinary with teams of scientists who inform and illuminate the different disciplinary perspectives of a problem.
At Earlham College, our small size, our existing network of collaboration and our interest in engaging innovation make our proposal feasible. We believe that this effort will build the framework upon which we will construct future multidisciplinary environmental studies at Earlham College. Indeed, there are many potential avenues of scientific inquiry toward which our concept may grow. It is our desire that the energized approach to undergraduate science education will prove useful outside of Earlham College. Thus, as we continually adapt and refine our concept, we look forward to being able to compile and export our effort to other liberal arts institutions that may benefit from it.
2) Objectives/goals for the proposed work.
The fundamental goal is the development of multidisciplinary curriculum modules and a related research program that incorporates computational methods to study an environmental problem of local significance. The project is designed to:
- Bridge the gap for students between scientific research and science education by incorporating research modules into several lower and upper division courses
- Increase student and faculty understanding of multidisciplinary use of field, laboratory, and computational methods to answer complex environmental problems
- Investigate the biogeochemical cycling of heavy metals (Pb, Zn, Cd, Cr, Cu and Ni) within a small-scale local watershed with particular emphasis on occurrence, speciation and phase associations as controls on mobility, bioavailabilty and toxicity
- Electrify student interest in the sciences by incorporating meaningful research into courses which address significant environmental problems within the local community
- Establish a new structural foundation for Earlham’s Environmental Sciences program by integrating our concept into the core courses of several disciplines
- Develop a workshop to teach faculty at other liberal arts colleges how to incorporate multidisciplinary research modules into their science courses
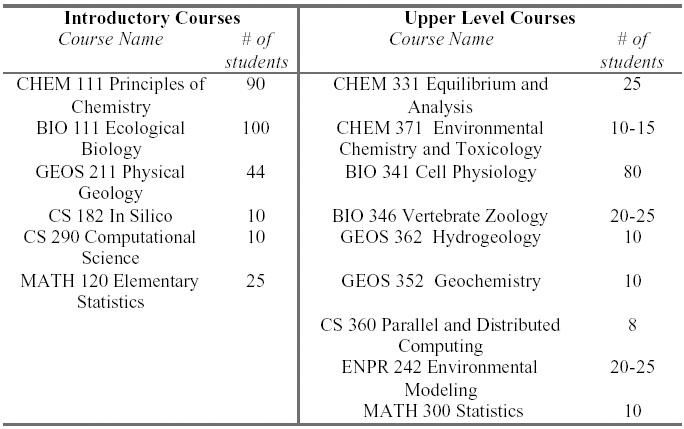
3) Project timeline keyed to the objectives/goals.
The implementation of this project begins with the development and refinement of the instrumentation and computer interfacing necessary to monitor field sites, as well as the development of the first sets of curriculum modules to be integrated into both lower and upper division science courses. It continues with the development and implementation of the remainder of the proposed curricular modules and with the implementation of advanced research projects. The project will culminate with a design for a faculty development workshop to disseminate the process and content of our concept to similar institutions. A final task will involve assessing the completion of the goals and objectives and preparing an evaluation of the effectiveness of the effort.
2007:
Spring:
- Preparation of a comprehensive plan to further guide design, implementation and assessment of the project.
- Development of instrumentation and interfacing for field monitoring equipment
- Development of CHEM 111 General Chemistry module
Summer:
- Development of CHEM 331Equilibrium and Analysis , GEOS 211Physical Geology, BIO 341 Cell Physiology and BIO 111 Ecological Biology course modules
- Deployment of field monitoring equipment
- Development of the data model and data storage for environmental and experimental data
- Development of the user interface for the environmental and experimental data
- Begin weekly summer student/faculty research gatherings
- Multidisciplinary collaborative research on metals in the environment
Fall:
- Implementation of CHEM 111 Principles of Chemistry, BIO 111 Ecological Biology, BIO 341 Cell Physiology and GEOS 211Physical Geology course modules
- Development of BIO 346 Vertebrtae Zoology, CS 360 Parallel and Distributed Computing, MATH 120 Elementary Statistics and GEOS 362 Hydrogeology course modules
2008:
Spring:
- Implementation of CHEM 331 Equilibrium and Analysis, BIO 346 Vertebrate Zoology, GEOS 362 Hydrogeology, MATH 120 Elementary Statistics, and CS 360 Parallel and Distributed Computing course modules
- Development of course modules for ENPR 242 Environmental Modeling
Summer:
- Formative Evaluation
- Development of course modules for CHEM 371 Environmental Chemistry, GEOS 352 Geochemistry, CS 182 In-silico
- Refinement of existing course modules
- Multidisciplinary collaborative research on metals in the environment (Biological Research:Tissue Levels of Heavy Metals in Selected Vertebrates from Springwood Lake, Correlation of Tissue Heavy Metal Concentrations with Histopathologic Evidence of Metal Intoxication, Expression of Metallothioneins in Tissues. Geochemistry: Fractionation, Treatment and Analyses of Soil and Sediment Mineral Fractions from Springwood Lake)
Fall:
- Implementation of CS 182 In-silico, ENPR 242 Environmental Modeling
- Implementation of multidisciplinary colloquium
2009:
Spring:
- Implementation of CHEM 371 Environmental Chemistry, and GEOS 352 Geochemistry course modules
Summer:
- NITLE workshop on integrating computational methods into the undergraduate science curriculum
- Papers and posters on the pedagogical aspects of this project
- Development of MATH 300 Statistics course module
- Evaluation
- Multidisciplinary collaborative research on metals in the environment
Fall:
- Critical evaluation and preparation of the final report
- Implementation of MATH 300 Statistics course module
4) Relation of the objectives to:
- the present state of knowledge in the field
Inquiry-based, multidisciplinary science education that involves computational technology is transforming the landscape of undergraduate learning in the natural sciences. Courses that are shaped around the practice and process of science rather than the rote mastery of facts and theories have been known to significantly enhance the learning of science in undergraduate courses (NRC, 1996; McConnaughay et al., 1999; Uno, 1999). Simply stated, students learn science best when they DO science. The importance of computational science in answering disciplinary research questions requires rethinking standard science curricula at the undergraduate level.
Currently, several national mandates call for this type of curriculum restructuring, and for good reason (e.g. Bio 2010 from the NRC, 2003; PITAC, 2005). Human societies place ever-increasing stresses upon the natural environment, with measurable destabilizing impacts upon complex ecosystems (NRC, 2003). In light of the reality that ecosystem health has direct and tangible influence on the sustainability of human culture, a more thorough understanding of ecosystem functioning is needed, both under vanishing natural conditions and after anthropogenic changes. In a 2001 report entitled "Grand Challenges in Environmental Science" a National Research Council committee sought to highlight the most significant environmental problems facing humanity. The first grand challenge identified is the need to improve our understanding of biogeochemical cycles, particularly with respect to anthropogenic disturbance of natural balances. In 2005, a National Research Council committee issued a report entitled "The Geological Record of Ecological Dynamics: Understanding the Biotic Effects of Future Environmental Change." In this report, the Committee emphasizes the importance of investments in multidisciplinary research as well as computational modeling. An illustration of the need to continue to develop and improve environmental models is the biotic ligand model (BLM), which is used to help predict toxicity of metals in the environment to a given species. There is still significant need for the development of BLMs to accurately model a wider range of organisms and metals, metal mixtures and to deal with chronic toxicity as most BLMs deal only with acute toxicity (Niyogi and Wood, 2004).
- work in progress by the project personnel under other support
There has been a long tradition of Earlham science faculty involvement in multidisciplinary and computational student/faculty research. In the last 20 years, faculty members in biology, geology and chemistry have been engaged in studies ranging from atmospheric measurements of mercury and aquatic ecosystem studies at the college's Dewar Lake Biological Research Station to determination of metal contamination in lake sediments. Across our science curriculum, a strong emphasis is placed on quantitative, analytical and research-based projects. Many of our research projects engage our student/faculty teams in multidisciplinary efforts; currently, our computer science faculty and students work with both biologists and chemists on computational projects (e.g. computational phylogenetic reconstruction and molecular dynamics simulations). For many years, our biology and chemistry departments have collaborated on a variety of research and curriculum projects, e.g. determination of atrazine concentration from agricultural runoff in local water sources and its effect on the physiological development of aquatic species.
The off-campus field site that was chosen for study is Springwood Lake (SWL) which is a small lake (~ 8 acres) located less than 2 miles from Earlham College. The watershed area that drains into Springwood Lake is characterized by land-uses that are historically associated with environmental contamination, including large-scale industrial/manufacturing facilities and unregulated and regulated solid waste landfills. Springwood Lake, presently owned by the City of Richmond, is a contaminated site listed ? This site has been studied by Mr. Parker and a student, Andrew Graham, who found relatively high levels of heavy metals in the sediment. As such, we believe this site needs further study. XXX I FIND IT QUITE INSULTING TO REPLACE MY DETAIL WITH THIS STUPID BLURB. WE HAPPEN TO KNOW A GREAT DEAL ABOUT SPRINGWOOD LAKE. WHY ACT LIKE WE DONT? (Maybe it's a space issue? This document is getting pretty long.)
Additionally, many of our science faculty have worked with state, municipal and public representatives to provide expert witness on local environmental issues. Examples of collaborative input to local issues include:
- Wood treatment plant XXX more here
- Hayes arboritum XXX more here
Mr Peck and his students are developing curriculum for computational science and parallel and distributed computing workshops under support from the National Computational Science Institute and the SuperComputing Education Program. These week long workshops are designed to teach undergraduate science faculty from a range of disciplines how they can use computational methods in their research and teaching.
- work in progress at other institutions
Many institutions have recognized the need for innovative approaches to science education at the undergraduate level. Carleton College has established an Interdisciplinary Science and Math Initiative (CISMI) aimed at integrating the physical sciences and mathematics in undergraduate courses and research projects. Additionally, Carleton College is currently working on ways to integrate computational modeling across their curriculum to enhance learning. Carleton is also emphasizing computational science at all levels of the curriculum, which is similar in scope to our proposed project. Trinity University is also focused on interdisciplinary faculty and student research as well as interdisciplinary curricular development with their recently funded Keck Center for Macromolecular Studies; however, Trinity’s program has a major focus on the integration of biology and chemistry, while our proposed program uses biology, chemistry, geosciences, mathematical, and computational science methods to explore environmental problems. Shippensburg University of Pennsylvania has implemented an Interdisciplinary Watershed Research Laboratory for field-based environmental laboratories. This project is similar in scope to our proposed project, but primarily integrates biology and geography/earth science, while we are proposing to involve more disciplinary perspectives as well as including computational modeling. Capital University in Columbus, OH has done extensive work on developing an undergradate curriculum and minor in computational science. With funding from the Keck Foundation they recently established the "Keck Undergraduate Computational Science Education Consortium". While Earlham's focus is clearly different from theirs they do offer a number of useful patterns and examples which we can learn from and build on when designing our own curriculum modules.
5) Concise description of methods and procedures for implementation and experimentation.
- Overview goes here
Curriculum modules will be incorporated into 6 introductory courses and 9 upper level courses in biology, chemistry, computer science, geosciences and mathematics. During the academic year, students taking courses that include these modules will be strongly encouraged to participate in a weekly, faculty facilitated seminar in which they will discuss their course experiences. At the end of each semester, students participating in courses that have these modules will be required to attend and present their group projects at a locally hosted poster session.
- Either by department or as a whole describe the summer research activities. Some of this is course module development and some "real" research. Developing local soil models, database (post grant).
Students will also be involved in multidisciplinary collaborative projects during the summer months. The summer research component will involve developing and testing curriculum modules in each summer (4 in summer 2007, 3 in summer 2008 and 1 in summer 2009). In addition, particularly in summer 2008 and 2009, students will have the opportunity to conduct more advanced research related to metals in the environment including analyses of metals in a variety of environmental matrices, descriptions and quantifications of food chains and computational modeling of rates of biomagnification of metals at higher trophic levels, performance of whole-soil hydraulic conductivity tests and determination of soil mineral reactivities, and computer modeling of biochemical and groundwater processes.
In biology, for example, some students involved in the curriculum modules will continue to work in summer research in 2008 and 2009. The biology course work will establish the populations and food-chains involved at Springwood Lake, while summer research will focus on the actual analysis of metals in representative organisms from the lake. In addition to samples saved from the course work discussed below, students will re-sample fish and turtles. Some fish will be sacrificed for analysis of metals in specific tissues, specifically gill, liver, kidney and gonad. Metal analyses will be performed by the Earlham chemistry department. Bioaccumulation and biomagnification at higher trophic levels will be assessed. Tissues will also be processed for microscopy and examined for histopathologic evidence of metal intoxication using routine histologic techniques which are currently being used at Earlham. We will also extend the Cell Physiology lab module into novel summer research designed to explore the correlation of the level of metallothionein expression in tissues with tissue metal levels and tissue histopathology. This work will involve Western blots and frozen tissue techniques, cryotomy and immunohistochemical techniques currently being done at Earlham and collaborating labs.
During each summer, there will be approximately six faculty and twelve students working on the curriculum module development and advanced research. All students participating in summer research will have two opportunities each week to discuss the multidisciplinary perspectives related to their projects: faculty from all departments will facilitate a weekly seminar and students will discuss their research projects in a student-led seminar.
- XXX Each courses' plan goes below. We need to show linkages between each of these courses where possible, both intra and inter departmental.
CURRICULUM MODULES
chemistry
Throughout our projects, site-specific data will be collected and models will be used to assess the fate and transport of metal contaminants.
We propose to incorporate a new environmental chemistry module in our general chemistry class (CHEM 111, typical enrollment of 90). This unit will introduce students to fate and transport modeling by measuring the distribution coefficient, Kd, which is a common parameter used to estimate the concentration and movement of metal pollutants in ground water. Kd is a measure of the extent of interactions between a pollutant and the soil matrix and is one of the keys to the understanding of the mobility and persistence of metals in the environment. A distribution coefficient for copper has previously been measured in a standardized soil material (Dunnivant, 2002), and the procedure can be adapted to soils collected from our study sites.
The module will be conducted over two laboratory periods. The first week will consist of a spectroscopy lab, where the students will be introduced to atomic and molecular absorption for the determination of the metal concentration in water, and to infrared spectroscopy for the characterization of the soil. In the second week, students will use atomic spectroscopy to determine Kd of a metal (copper in year 1, and additional metals in subsequent years) in both standard soils, and soils collected from both our field and test sites.
The distribution coefficient for metal contaminants varies greatly with experimental conditions, both of the soil and the aqueous system (pH, ionic strength, concentrations of pollutants, etc.). This variability will be illustrated by looking at the effect of pH on Kd for the soils investigated. The results will be used to discuss such environmental issues as acid rain and metal mobilization. Using the Kd results obtained in the laboratory and some simple assumptions, students will calculate a retardation factor(R) for the movement of the metal through the soil/water system (R = (1 + (pb/ne) * Kd)), where pb is the bulk density of the soil and ne is the soil porosity at saturation) . The effects of the chemical changes on Kd and on retardation factor will be modeled using a spreadsheet based model. The soil Kd results will also be integrated in a database for use in transport modeling, and could be further studied in the student research projects in the Equilibrium and Analysis class (CHEM 331).
The Equilibrium and Analysis course (CHEM 331) is a sophomore level course with an approximate enrollment of 25 students per year. The module for this course will be conducted during a two-week laboratory (four lab meetings) and will use diffusive gradients in thin films (DGT) to determine the speciation of metals (Cu, Ni and Zn) in both simulated laboratory solutions as well as water samples obtained both from the field site and the off-campus site. The speciation results of the DGT study will be compared to the results obtained with the speciation model Visual MINTEQ, a freeware version of the program released by the United States Environmental Protection Agency (USEPA).
This experiment will replace two of our current labs designed to expose students to atomic spectroscopy. It will accomplish the same pedagogical goal of teaching students how to prepare samples and standards for atomic analysis, as well as provide them with hands-on experience with the equipment necessary for spectroscopic analyses. Moreover, students will now also learn about ion exchange, metal speciation, the environmental importance of bioavailability, as well as the power of computational modeling.
In DGT, metals are accumulated on an ion exchange resin imbedded in a hydrogel and covered with a diffusion layer of a different hydrogel and a filter (Zhang and Davison, 1995). In this technique, the transport of metal ions to the resin occurs only by molecular diffusion (ref), resulting in a direct linear relationship between the metal mass accumulated and the time of deployment (see figs 1 and 2 – from Zhang and Davison, 1995))
The advantages of DGT include its simplicity, relative independence from hydrodynamics of the system, multi-elemental capability and its inherent pre-concentration capabilities, which enables the measurement of even extremely small concentrations in solution. With this technique, only the labile forms of the metals in solution (i.e. the inorganic free metal forms and organic labile complexes) will be measured. In addition, since the labile inorganic and the labile organic complexes have different diffusion coefficients in the hydrogels, the concentration of each type of species can be determined by this method. Zhang and Davison (2000, 2001) have shown that by varying the properties of the diffusion hydrogel, both the organic and inorganic metal concentrations can be obtained. In addition, the authors demonstrated that a single DGT device, with a very restrictive pore size, could measure the labile inorganic concentration with only a small correction factor. When using a single DGT device, the total concentration of a metal in the solution must be determined, and the difference between that total value and the DGT value will represent the organically bound metal. Because of their well characterized nature, we will purchase the DGT device components from DGT Research Ltd during the first few years of implementation. As part of the summer research, we intend to characterize the diffusion coefficients of various metals and organic species (humic and fulvic acids) in polymers that we will synthesize. In addition, resins other than the standard Chelex (used to bind many +2 and +3 ions) can be explored for selectivity of other metals or oxidation states that are bound tightly to Chelex.
In practice, these devices are deployed into a well-stirred solution for times ranging from hours to days. After this time, the device is disassembled and the metals are then eluted from the resin using a nitric acid solution and analyzed using either graphite furnace atomic absorption spectroscopy (GFAAS) or inductively coupled plasma atomic emission spectroscopy (ICP-AES).
Three different systems will be tested using this method: a laboratory system with known parameters, a water sample from our field site, and a water sample from our remote site (Springwood Lake). These water samples can be tested both for endogenous metals as well as spiked laboratory samples. The water samples from the field site and remote site will be characterized by the teaching assistants for the additional parameters (such as anion concentration) required for Visual MINTEQ modeling, but not measured directly by the students in the course .
Previous literature indicates that DGT can accurately predict speciation under a variety of conditions if the water parameters are well characterized (refs). In particular, it is important to know precisely not just the DOC (Dissolved Organic Carbon), but the percentage of DOC that is humic acid vs. fulvic acid. While both humic acid and fulvic acid complexes diffuse much more slowly than the inorganic form, humic acid complexes diffuse about twice as slowly as fulvic acid complexes. In addition, both acid concentrations have a large impact on the speciation model predictions (Zhang and Davison, 2000). The effect of varying these parameters on the fit of the model to the experimental data will be explored.
This experiment will be done over four lab periods. During the first lab period, the students will set up the water samples with the DGT devices and allow them to incubate until the following laboratory period (48 hours). They will also be introduced to the trace metal analysis equipment (GFAAS and ICP-AES) and will prepare and analyze standards and samples for major cations using ICP-AES. During the second lab period, students will elute the devices and analyze them using the appropriate method. During the third and fourth lab period, the students will utilize the Visual MINTEQ program to calculate the speciation and then compare with the measured results. As part of the laboratory samples, students can explore the effects of pH, dissolved organic content and ionic strength on both the DGT results as well as the agreement between the model calculations and the DGT measurement. Students will also be provided with data from the Principles of Chemistry class on Kd and its variation with the same parameters. The effects both of transport and bioavailability on the ultimate toxicity of a given metal will be discussed.
The Environmental Chemistry and Toxicology course (CHEM 371) is a new course that will be introduced in the spring of 2007. This course will be an upper level chemistry course with a prerequisite of Equilibrium and Analysis. The expected enrollment will be 10-15 students.Two modules will be developed for this course and those two modules will comprise 8 weeks of the laboratory portion of this course. The learning outcomes for the students include understanding the complexity of metal speciation in water, proficiency in the operation of the many complex instruments needed (e.g. atomic methods, ion chromatography), understanding the basics of a toxicological study, and an appreciation for both the power but also the limitations of computational methods.
As an extension to the work performed in Equilibrium and Analysis, the first module will involve the in situ deployment of DGT devices at both our field site and our remote site. This module will take four weeks to complete. In the first three weeks, students will learn about water sampling and will deploy the DGT devices. Data on temperature, pH, dissolved organic carbon, alkalinity, as well as major cations (Ca2+, Mg2+, Na+ and K+), major anions (SO42- and Cl-), and sulfide concentrations will be collected for both sites. In addition, further characterization of the dissolved organic content for each site will be performed in order to accurately quantify the amount of humic acid and fulvic acid present (this data will then be provided to the Equilibrium and Analysis course to help in their calculations). For this model, we will deploy several DGT devices, each of them corresponding to a different diffusion layer hydrogel. The data obtained will provide a direct measure of the metal speciation. During the fourth week, Visual MINTEQ will also be used to model each metal speciation.
A second module will involve the use of a model organism, Daphnia magna, to monitor toxicity of a metal in various water samples and the use of the biotic ligand model (BLM) to predict toxicity under the same conditions. This module will also encompass four weeks of lab. Toxicity testing with a specific organism is the most accurate method of determining the toxicity of a metal in the environment, but also the most time consuming. The biotic ligand model, which treats the organism of interest as a ligand which is competing for metal binding with the other ligands in the solution, is of great interest because of its ability to greatly simplify the measurement of potential toxicity.
Well characterized water samples from our two test sites, as well as laboratory samples for various metals will be used in the study. Daphnia magna, also called water fleas, are crustaceans commonly used in environmental toxicity studies. These are easy to handle and grow as well as inexpensive. Using these organisms, students will first construct toxicity curves for known metals (Cu, Cd, Zn) in synthetic water solutions with controlled parameters. These studies will then be repeated using water from the three systems described above. The results will then be compared to those predicted by the BLM (ref) to demonstrate the usefulness of such a model as well as help students understand how they are constructed.
biology
The ecological impact of heavy metal pollution at Springwood Lake will be explored through modules within two field biology classes and through summer research. We propose to develop a module for Biology 111, Ecological Biology. This is an introductory level course, with approximately 100 students annually. Non-science majors typically represent 50 to 65% of the class size. Students will help inventory the biota of the lake in a sampling lab, share data, interpret data, and be introduced to population modeling computation.
Learning outcomes include:
- Gaining an appreciation for the interrelationships and natural history dynamics of ecological communities.
- Understanding the importance of quantitative techniques and reasoning in the description and study of complex ecosystems.
- Gaining the ability to critically evaluate sampling protocol efficacy and the quality of quantitative data.
In this module, each lab group in EcoBio will make a field trip to Springwood Lake and learn aquatic biota sampling techniques. Plants will be sampled manually; invertebrates by plankton tows, nets, and dredges; and vertebrates by seine or fyke nets. Students will work in groups of four or five and each group will gather higher taxon-specific data such as mensural and meristic measurements, age estimations, population density and biomass calculations. Portions of samples will also be preserved for metal analysis by the chemistry department during summer research. Groups will share data and an accurate biotic inventory of the lake will emerge.
This module would be introduced in the Fall of 2007 and continue, allowing us to track changes in populations which will be vital information for assessing the significance of heavy metal pollution at the lake.
Biotic inventory work and food-chain construction will be continued in a module to be developed for Biology 346, Vertebrate Zoology. This is an upper-level course with a typical enrollment of 20-25 students.
Learning outcomes for this module include:
- Learning the theoretical concepts as well as the field techniques of population ecology of vertebrates.
- Learning about vertebrates in aquatic ecosystems and aquatic toxicology and how geology and chemistry are vital to understanding these fields.
- Being exposed to the application of contemporary computational methods used in vertebrate population modeling.
In this module, students will use mark-recapture studies (using injection of passive integrated transponders [PIT tags]) to estimate population sizes and standing crop biomass of macrovertebrates (fish and turtles). Gut content analysis (by dissection for invertebrates, and non-destructive stomach flushing of vertebrates) will be used to determine food chains. Blood and/or tissue samples (non-destructive whenever possible) will be saved for analysis of metals during summer research, and these values will be related to the trophic ecology of individual species. We would also do sampling of tissues of these same organisms in other county lakes as reference values for this general region.
This module will be introduced in the Spring of 2008, and repeated annually, with special emphasis on following tagged animals and investigating evidence for bioaccumulation of metals in these animals.
Keck-Cell Phys Module
A laboratory module will be developed for Biology 341, Cell Physiology. This is a sophomore level course which typically has an enrollment of approximately sixty students. Over the course of the semester, students will investigate the effects of heavy metals on the expression of stress proteins in fish red blood cells and explore the roles of chemistry, geosciences, math and computer sciences in toxicology.
Because they are nucleated and capable of transcription, translation, and protein synthesis, fish red blood cells are a valuable model for studying cellular responses to a variety of stresses, including heavy metal toxicity (Currie and Tufts, 1997). The heat shock proteins, HSPs, and metallothioneins, MTs, are two families of stress-induced proteins frequently studied in heavy metal toxicology. Fish red blood cells reliably respond to heavy metal stress by the expression of HSPs, and in some cases do so in a graded, dose-dependent manner, while the expression of MTs is more variable and less well studied (Fulladosa, et al, 2006.) In this lab, fish red blood cells will be used to investigate the affect of metals on stress protein expression, and they will test hypotheses about what physical and chemical properties of aquatic environments affect bioavailability and bioabsorption of metals.
Learning outcomes will include: • Gaining an understanding of how cells and organisms respond to heavy metal intoxication. • Understanding how bioavailability of heavy metals is a function of water characteristics and how chemistry and hydrogeology play important roles in toxicology. • Gaining experience in experimental design, multivariate statistics, spectroscopy, protein quantification, and Western blot analysis. • Being introduced to bioinformatics.
In weeks 1-3 of this lab module, students will use the science library and technology resources to explore primary and secondary literature on the topics of research models, including fish red blood cells, for studying cell physiology and toxicology; HSPs and MTs; aquatic toxicology; bioavailability; and heavy metal bioabsorption and toxic effects. After this introductory material, students will design their experiment protocols.
They may choose what metals to challenge cells with; and they choose what chemical or physical variables to add to the protocol to test hypotheses about what factors affect bioavailability/bioabsorption (see Methods below.) Variables the students might explore include nitrates, sulfates, organic matter, anions, acid or alkaline conditions or variable temperature.
During this period they will also construct standard curves for protein quantification on spectrophotometers with bovine serum albumin and the Bradford method.
In weeks 4-7, the wet lab will be conducted. We will purchase juvenile or adult rainbow trout, Oncorhynchus mykiss from a regional supplier. Ideally, the trout will have previously been kept in Earlham aquaria for an acclimation period of one month. Trout will be anesthetized in buffered 3-aminobenzoic acid ethyl ester (MS-222, Sigma) and blood drawn from the caudal vein. The red blood cells will be separated by centrifugation and then incubated in Hank’s Balanced Salts Solution prepared with known amounts of CdCl2, PbCl2, or K2Cr2O7, either alone or in combination with the chemical or physical variables chosen to assess bioavailability/bioabsorption. Cells will be incubated at 20oC for 2 hours and samples prepared for protein analysis by lysis, centrifugation and supernatant collection. Total protein will be determined by the Bradford method, and gel electrophoresis and Western blot will be used to detect HSP70 using murine anti-HSP70 antibody (Sigma). Students will be able to semi-quantitatively analyze changes in HSP70 expression among the various groups. MT expression will be detected by dot-blot analysis using murine anti-MT antibody (Sigma.)Groups will share their data with the entire class.
Weeks 8-12 will involve data interpretation and multivariate statistical analysis, as well as the bioinformatics portion of the module. A member of Earlham’s math department will help with the instruction of the multivariate statistics. Biology students are first introduced to statistics in Ecological Biology and this represents an important step in the progression of their exposure to statistics.
The bioinformatics portion of this module will center around the metallothionein family of proteins. Metallothioneins (MTs) are polypeptides, and like heat shock proteins, they are produced in response to metal intoxication in a number of organisms and models, including fish red blood cells (Bauman, et al, 1993.) MTs are known to help protect cells against cadmium toxicity and they bind a variety of metals via abundant cysteine residues, which are highly conserved across phyla. Students will use Biology Workbench, SwissProt, and ClustalW to construct gene trees and phylogenies using the MT family of genes. They will also explore MT structure, folding, function and molecular evolution using PDB, Kegg and ConSurf.
This module is a modification of the current Cell Physiology laboratory using similar if not identical methods and equipment. In the current lab, students investigate the expression of HSP70 in hemolymph of the tobacco hornworn, Manduca sexta, as induced by a variety of stressors. This has been a very successful lab with good results. The initial literature search portion as well has been an important component of Cell Physiology. Earlham has long-excelled in science library and science information technology pedagogy. This particular module will be developed in the summer of 2007 with summer research students performing all aspects of the lab, including preparation, writing a lab manual, and trouble-shooting. It will be the first time that multivariate statistics and bioinformatics will be taught in this course.
geology
The contribution of the Geosciences Department will be to inmplement course modules in intro-level Physical Geology and upper-level Hydrogeology and Geochemistry courses. Additionally, the soils and sediment samples that will be analyzed during the Geochemistry module require extensive fractionation and treatment. Soil and sediment will be processed during the summer of 2008.
Lower-Level Course Module: GEOS 211 Physical Geology
Physical Geology at Earlham is an introductory-level course that is taken by both science and non-science majors. Students in this course who are non-science majors generally lack confidence in their ability to “do†science and have had little to no exposure to an inquiry-based science classroom. In this course module, students will apply fundamental geologic methods of analysis to an environmental project. By the end of this module, students will be able to:
- Use web-based GIS to display and organize data relevant to the characterization of the project site.
- Use field and laboratory observations to describe the geology of the project site.
- Organize and analyze geochemical data to display the concentration distribution of heavy metals in lake bottom sediments at the project site.
- Establish a chronology of heavy metal loading to the project basin via interpretation of heavy metal stratigraphy.
- Create a scientific report synthesizing the results of the project and suggesting areas for further study.
This module will use the final four laboratory sessions in Physical Geology. Students will have a basic background in geology and will be able to apply that knowledge to the local area. Each laboratory section has a maximum of twenty-two students, with one professor and one upper-level undergraduate teaching assistant.
In the initial week of the course module, students will be assigned readings and worksheets that focus on the general problem of metals in the environment with emphasis on lake sediments as pollutant archives. Readings will be keyed to discussions of the hydrologic cycle with an emphasis placed on the connection between groundwater flow and subsurface geology. Students will begin to learn how to use web-based GIS to create displays of the study area.
A field trip to the project site will occur in the second week of the project. Students will examine the geology and hydrology of the project site (Springwood Lake) and participate in a demonstration of sampling a sediment core from the lake.
After students have seen the site locality and have done some initial reading, students will observe and describe a suite of sediment cores to determine terms of sediment composition, texture, color, sorting, fabric and sedimentological characteristics.
As a final component to the module, students will be given geochemical data keyed to the cores described in the previous week's lab (geochemical data will have been collected by upper-level geochemistry students or will have been collected as part of a summer research project). Students will be required to plot and analyze this data and make interpretations about the concentrations of heavy metals in Springwood Lake over time as a result of their analysis. Students will then write a full scientific report of this project and share the results with other introductory-level science students working on different aspects of this project.
The course module will require some rearrangement of existing laboratory topics. The site field trip will replace an existing field trip that focuses on the Richmond, Indiana drinking water supply. An existing lab that is entirely devoted to sedimentary rock description will be modified and incorporated into another rock lab, with the sediment core descriptions replacing it. A current lab that introduces GPS and GIS to students will be modified so that it becomes the laboratory session in which students read about the environmental problem and learn to use Web-based GIS. GPS techniques will be used in the field lab. A current lab offered in this course gives students experience with data analysis; however, the data used is related to plate motions over time and is part of a lab on global tectonics. The same techniques of analysis can be taught using the geochemical data related to Springwood Lake metals and will provide a real-life example of collecting and analyzing data related to an environmental problem.
Upper-Level Course Module: GEOS 362 Hydrogeology
Hydrogeology at Earlham is taught with an emphasis on practical application of theoretical concepts. Two course modules will be developed for hydrogeology; one for each of the project sites. These course modules will enrich student comprehension of the significance of ground water/surface water interaction in the vicinity of the project sites and will develop student capabilities for collecting, analyzing, displaying and interpreting ground water data. In addition, students will interact with several types of ground water flow models.
At the on-campus field site, students will be active participants in designing, installing and managing data collection from ground water monitoring devices. instrumentation instrumenting the
At the conclusion of these modules students will be able to:
- Evaluate data collection requirements (number and spacing of piezometers, monitoring wells)Prepare geologic and hydrostratigraphic unit cross-sections from well-boring data.
- Obtain laboratory measurements of whole soil porosity for each hydrostratigraphic layer at each of the project sites.
- Collect, tabulate and display ground water elevation data and prepare detailed maps of potentiometric surfaces.
- Determine horizontal hydraulic conductivity values for saturated media at each project area.
- Computationally analyze potentiometric and porosity data, slug test and constant-head discharge data to calculate rates of ground water flow, estimate hydraulic conductivity values, and determine time-drawdown and distance drawdown behavior as a means of establishing aquifer parameters transmissivity (T) and storativity (S).
The first module aims to teach students how to assess the type and the amount of data they will need in order to characterize the behavior of ground water within the study volume. They will also be asked to determine how the data they collect can be used to note fluxes across the study volume and changes in ground water behavior over time. Student inquiry will be guided by classroom learning on the fundamentals of ground water flow in porous media and field demonstrations on the design and installation of ground water monitoring instruments (multi-level piezometers and monitoring wells). Monitoring instrumentation will be hand-installed - by Hydrogeology students - within the on-campus research plot. Wells will be placed inside industrial sewer pipe advanced vertically to a depth of 6 or more feet. This pipe, used as an outer casing to prevent sidewall collapse, will be advanced by the weight of students standing (and jumping) on top of it within a safety scaffolding. The soil inside the pipe will be continuously removed by an over-sized bucket auger to facilitate casing penetration. A 4" I.D. PVC monitoring well will be installed in the open borehole with a section of slotted well-screen at the bottom. The casing will slowly be jacked back out of the borehole by use of an engine hoist. As the casing is slowly removed, well-sorted pool filter sand will be added to spill out the bottom of the casing and surround the well-screen. The sand pack, added to a level 6 inches above the top of the screen, will be capped with pelletized bentonite, a Na-montmorillonite expandable clay. Teh well will be completed with a steel-jacket lockable cover cemented to the ground with 2 80 # bags of Portland cement. Several wells will be installed in this manner. Multilevel piezometers will be installed by advancing a 1" I.D. PVC pipes to specified depths with a sledge hammer. Pipes will be fitted with conical drive points to facilitate installation; points will be hammer out at the design depth to permit communication of the piezometer with the ground water system.
After installation, wells and piezometers will be developed (bailed or pumped) to remove muds suspended by installation disturbance. The top of casing elevations will be surveyed with a transit and stadial rod to the nearest 0.01 foot. Students will collect weekly ground water elevation data using a Solinst Watermark Water Level Meter. Aquifer hydraulic conductivity values will be obtained by running constant head slug tests according to the method of Hvorslev (1951, described in Domenico and Schwartz, 1998). Aquifer parameters T and S will by acquired by means of a 24 hour constant-discharge pumping test. Data from the slug tests and the pump tests will be computationally evaluated using AQTESOLV, version 3.5 (5 licenses owned by the Geosciences Department). After hydraulic testing, wells will periodically be sampled for water qulaity. Wells will be pumped or bailed to remove a minimum of 3 well volumes and measurements of ground water temperature, conductivity, pH and oxidation/reduction potential (ORP) will be collected using a WTW 340i Multitester. Ground water samples will be collected and analyzed for common anions and cations and EPA Primary and Secondary standards. These data will form the basis for student reports describing the quantity and quality of ground water passing through the study plot.
The second module will be developed so as to challenge students to manage, organize, analyze and interpret extant ground water elevation data associated with the multiple ground water monitoring wells in the industrial areas surrounding Springwood Lake. These data are compiled by the Voluntary Remediation Program (VRP) at the Indiana Department of Environmental Management (IDEM) and are public records subject to open access. Students will be divided into small groups (3 or 4). Each group will be taken on a field trip to visit the Indiana Department of Environmental Management. Students will be instructed in the proper methods for acquiring publicly available records. They will be assigned the task of obtaining a complete monitoing cycle worth of ground water elevation data. Once data is in hand, students will enter ground water elevation and well location data into spreadsheets. The spreadsheets will be saved to DatabaseIV (.dbf) format and will be imported into ArcGIS 9.1 so that the data may be contoured and shaded to produced a 3D map of the water table surface. Potentiometric maps will be created from different snapshots in time be each group of students. Students will be prepare a report describing the variation in the ground water system over time and will integrate subsurface data (soil stratigraphy from well logs, data from geophysical surveys, etc.)to complete characterization of the ground water flow system communicating downgradient with Springwood Lake.
Completion of the second module will spawn a number of new questions. Examples include: Does calculated ground water flow behavior predict the volume of springflow entering Springwood Lake? Do subsurface heterogeneities modify or control flow behavior? Can ground water flow characteristics account for ground water chemistry discharging as springflow or as baseflow into the lake? New questions may then be incorporated into continuation of the hydrogeological investigation or they may form the basis for additional grant proposals after the 3 year term of the Keck grant.
Upper-Level Course Module: GEOS 352 Geochemistry
A geochemistry course module will be developed around a detailed investigation of the tiny crystalline solids (minerals) that govern the physical and chemical properties of soils and sediments. The objective of this module will be to characterize the adsorptive mechanisms governing association of cations with these solid phases. This will be achieved by determining the relative contribution of colloidal silicates, humus, silica and iron oxides contained therein (Sposito, 1984). The methodology involves a complicated series of preparational steps to isolate and identify specific mineral fractions from soil and sediment. The methods are described in detail in Dixon and White (1996) with robust theoretical underpinning in Dixon and Weed (1989) and Sposito (1984, 1989). Because these material preparations take a long time to complete, they do lend themselves readily to treatment in a module. Rather, soil and sediment preparation and treatment will be accomplished by several students working of the course of a summer. A synopsis of the treatment train is as follows:
- Air-dry a minimum of 25 g of clayey soil or 100 g of sandy soil (to ensure a minimum of 1.5 g of both the coarse and fine clay fraction).
- Analyze a bulk untreated sample via randomly-oriented powder mount X-Ray Diffraction (XRD)
- Determine moisture content in a representative 5 g sample heated overnight at 105oC (this removes adsorbed water without influencing structural water).
- Remove binding agents (carbonates, organic matter, iron oxides) and flocculating multivalent cations.
- Carbonates and multivalent cations removed by reaction with pH 5 sodium acetate (NaOAc) buffer solution heated to 90oC, then centrifuged down. Repeat several times. NaOAc also replaces Ca2+ and Mg2+ cations at exchange sites.
- Organic matter and MnO2 are removed by reaction with 30% H2O2 solution in a pH 5.0 NaOAc buffer for several hours at room temperature
computer science
Computer Science has four distinct roles in this project. In general, working with scientists from other disciplines on computational methods has been a focus of our department for some time now. We have experience with the pedagogical, research, and operational aspects of computational science and the high performance computing gear that support it.
First, we will be designing and building field-deployable monitoring systems for use at the on-campus study plot. These will be small, solar powered, single-board computer based units with the capability to monitor, record, and up-load temperature, pH (digital), conductivity, redox potential, pressure, and nitrate levels. This will build on exisiting work that the Hardware Interfacing Project, one of our student/faculty applied computer science groups, has done with field-deployable weather stations.
Second, we will be working with faculty in Chemistry, Geosciences, Biology, and Mathematics to help them design and implement the computational components of their curriculum modules. Our experience developing curriculum for the National Computational Science Institute and the SuperComputing Education Program will prove valuable here.
Third, working with the other scientists we will design the data model for this project and then implement it using a relational database management system. The Green Science Group, another student/faculty applied science group, has developed user interfaces for energy and weather data, we plan to work with them to develop the appropriate interfaces, classroom and research, for the data being collected as part of this project.
Fourth, we will be adding a new curriculum module, more heavily based in science (in this case chemistry and physics), to Parallel and Distributed Programming, one of our upper level classes. Parallel and distributed programming techniques are at the heart of most of the scientific software kernels used for computational science and modeling. Problems in this domain tend to be computationally intensive, thereby requiring the use of clusters, grids, and other high performance computing platforms. The effective use of parallel and distributed algorithms, combined with high performance computing gear, enables us to reduce the time to science for educators and researchers in biology, chemistry, etc.
Science for geeks as opposed to technology for nerds.
For Chemistry, Biology, Mathematics, and Geoscience the challenges at hand are a) teach their students a new, third framework for scientific inquiry, computational methods, and b) show them how multi-disciplinary teams of scientists approach large, complex problems. For Computer Science the challenges are slightly different. While we share the need to show them how multi-disciplinary teams of scientists work we don't need to spend very much energy on computational methods. On the other hand we do need to show them how the tools of their trade are employed within chemistry, biology, etc. and give them first-hand experience doing experiments with both in-vitro and in-silico components.
For a number of years the Cluster Computing Group at Earlham has been working in the area of molecular dynamics, specifically protein folding. In this context we have done a significant amount of work with GROMACS, a popular open-source molecular dynamics package which is typically used with large bio-molecules such as proteins. Our posters and papers, and collaborative work with the Pande Lab at Stanford University (home of the Folding@Home project), cover a variety of topics. For this project we propose to develop a curriculum module which extends our work in this area into the underlying chemistry and biology. Working with Dr's Matlack and M. Deibel we will perform simulations of protein folding in the presence of metals. (XXX more science here.) The inclusion of this module into the Parallel and Distributed Computing class will give computer science students the opportunity to learn about the underlying science and see how the simulation is developed from nature and how the in-silico and in-vitro experiments relate to and inform each other.
Validation, i.e. is it the correct model, and verification, i.e. is the model implemented correctly, are two critical topics to cover in the context of computational science education. Scientists of all disciplines must understand the limitations associated with simulating natural processes in-silico and how to validate and verify the computational models they are working with. Our plan is to explicity discuss these aspects in each course when a particular model is introduced in a curriculum module. For some topics, e.g. groundwater flow modeling, we can perform experiments that give students first-hand experience with the verification process. In this case we will use table-top groundwater flow simulators which contain soil strata from our back-campus study plot. Working in a computer equipped soils lab students will be able to develop and refine a computational model of contaminent transport using the table-top simulator for verification.
Computer Science is currently working on the development of two new courses germane to this proposal, In-silico and Introduction to Computational Science.
In-silico, developed and taught by Dr. James Rogers, will be an introduction to simulating the natural world using spreadsheets and other relatively simple computational tools. This course will be designed for first-year students, both as an attractor to the sciences generally and as a foundation on which more sophisticated computational approaches can be developed later in the curriculum.
Introduction to Computational Science, developed and taught by Mr. Charles Peck, is also designed to be accessible to lower division students although here we expect to attract a high percentage of potential science majors from a variety of disciplines. The goals and objectives of this course are:
- To introduce the concepts of computational science, modeling, and simulation.
- To introduce and develop skills with the tools commonly employed within computational science, e.g. symbolic, numeric, and graphical computational packages.
- To facilitate collaborative learning through regular small group projects which have students from different majors working together.
In both cases the courses will be structured in a way such that students taking either or both of them, who go on to take any of the upper-level science courses with computational modules which are a part of this proposal, will be well prepared to extend their learning in those areas.
mathematics
Math 120, Elementary Statistics, is a general education course taught each semester in which students are introduced to the key notions of statistics: descriptive statistics and inference testing. We will make use of the "metals in the environment" data sets in teaching students how to do typical tasks of descriptive statistics: measures of central tendency, measures of dispersion, and construction and interpretation of graphical displays of statistical information (univariate and bivariate data). Use of data sets from Springwood Lake and our back-campus field site will help students grasp the differences in origin and potential uses between observational and experimental data. Probably the greatest advantage of using this data is that it will be "real" to the students. They will know the sites (and potentially the historical context of those sites) where the data is collected, and the students involved in the experimental design and data collection.
Math 300, Statistics, is a calculus-based introductory course which we teach every other fall semester. We would make similar use of the data sets as for Math 120.
EnPr 242, Environmental Models, is taught each fall and is required for all students who are earning a major or a minor in either environmental science or environmental studies. This course is taught by a mathematics professor and includes application modules involving biology, chemistry, computer science, geology, and mathematics. The "metals in the environment" data will be used with the module on the chemistry of hazardous materials. The wells at the backcampus site will provide data that will be very useful for the module which introduces the organization, analysis and interpolation of scatted spatial data. And the aquatic biota population data from Springwood Lake will be integrated into the population biology module. As with statistics courses, the use of Earlham-related data will motivate students in their use of linear algebra and geometry to model environmental situations.
6) Technical problems that may be encountered and how they will be addressed.
There are several types of technical problems that could be encountered during this project. We are implementing many new methods, instrumentation and software as part of both course module development and our summer research projects. The methods will generally be adapted from the literature, but will require significant modification and testing to be suitable for use in an undergraduate course where students are still learning proper laboratory techniques.
While the instrumentation that we will implement uses sensor technology which is new to us, the idea of field-deployable data collection and monitoring gear is not new to Earlham. The Computer Science department, in the form of the Hardware Interfacing Project, has set up and maintains weather stations and other scientific gear in field settings. Those experiences will help us overcome any obstacles the new devices may present.
For most of the modeling applications we will be utilizing open-source software packages. Many commissions and study panels have highlighted the value of open-source software in the context of scientific inquiry. One of the most important attributes of open-source software for this proposal is the ability to "open the hood", either to show students the core algorithms or to make adjustments to the code.
7) Roles of all key project personnel.
Michael Deibel - As part of this project, Dr. M. Deibel will help develop course modules for environmental chemistry and equilibrium and analysis. In addition, he will conduct research with students and other faculty to analyze water, soil and biological samples for various metals. As Project Leader, Dr. M.Deibel will help coordinate development and implemenation of all modules and research and will be responsible for putting together the yearly and final reports.
Corinne Deibel - Dr. C. Deibel will participate in the development and implementation of the teaching modules in general and analytical chemistry. She will conduct research with students and other faculty to analyze water, soil and biological samples for various metals.
John Iverson - Dr. Iverson will develop and implement the module for Vertebrate Zoology. He will also train students and Dr. Matlack in techniques to be used in summer research and in course modules: macrovertebrate sampling, tag and re-capture techniques, turtle handling, etc.
Mic Jackson - Dr. Jackson will develop and implement course modules for Elementary Statistics, Environmental Modeling, and Statistics. He will also work with other Earlham researchers to insure that data collection and organization is done in a way to ease the use of data in classroom assignments.
David Matlack - Dr. Matlack will conduct summer biological research with students and develop and implement the module for Cell Physiology.
Ron Parker - Mr. Parker will develop and implement course modules for Hydrogeology and Geochemistry.
Charlie Peck - Mr. Peck will work with students to design, build, and deploy the field monitoring equipment to be used at the two study sites. Mr. Peck will work closely with the other faculty to develop the computational components for each of the course modules described in this proposal and to design and implement the data model, data storage, and user interface to the long-term environmental data to be collected and analyzed as part of this work. Working with colleagues in the Computer Science Department, he will develop curriculum modules for In-silico, Introduction to Computational Science, and Parallel and Distributed Computing.
Meg Streepey - Dr. Streepey will develop and implement the course module for Physical Geology. She will also direct the management and coordination of the multidisciplinary student seminar programs.
Lori Watson - Dr. Watson will assist in the development and implementation of course modules for Principles of Chemistry. In addition, she will coordinate the program assessments during years 2 and 3 of the grant.
8) Organization chart of key project personnel.
.
9) Description of facilities, equipment and resources available for the project.
The science complex at Earlham consists of three interconnected buildings, Dennis Hall (Computer Science, Geology, Physics, and Mathematics), Noyes Hall (Science Library, large computer lab) and Stanley Hall (Biology and Chemistry) with a net square footage of 76,000. .
The laboratory portion of the chemistry modules will utilize two laboratories (one for general chemistry/equilibrium and analysis and a separate lab for environmental chemistry). These labs have a total square footage of 2950 (1719 and 1230 ft2, respectivly) and a combined total of 11 hoods. Each lab has benchspace for approximately 20 students.
Analysis of metals will be conducted on two separate instruments: an inductively coupled atomic emission spectrometer (ICP-AES) and a combination flame/graphite furnace atomic absorption spectrometer (GFAAS). The ICP-AES (Perkin Elmer Optima 4100DV) is a dual view, multi-element analyzer capable of analyzing up to 50 elements in under a minute. We have also recently installed an ultrasonic nebulizer (CETAC U-5000-AT), which will increase our sensitivity by a factor of 10. The GFAAS (Perkin Elmer AAnalyst 800) is a state of the art transverse heated graphite tube system with Zeeman background correction. This instrument will be utilized in GFAAS mode to analyze metals when the detection limits of ICP-AES are insufficient for the levels present in our samples. It will be utilized in flame mode when analyzing major elements such as calcium or magnesium. Both instruments are fully automated (allowing the analysis to continue even after the 3 hour lab has ended) and utilize the same software platform (which will simplify student training).
Prior to analysis by the methods listed above, solid samples must be converted into a homogenous aqueous form. Our current microwave digestion system (CEM MDS-2000) has both temperature and pressure control and will be useful in method development for digestion protocols. It is, however, limited in sample throughput to 12 vessels.
Three different biology teaching laboratories will be used for the course modules. Each lab is approximately 1200 square feet, with bench space for 24 students. Equipment to be used includes dissecting microscopes, compound microscopes, spectrophotometers, UV spectrophotometers, mini-protein gel electrophoresis units, mini trans-blot cells, microcentrifuge, 6 computer stations for statistical analysis, E-gel units, refrigerators, ultra-low freezer, and environmental chambers. Summer research in biology will be conducted in the faculty research area which is 1500 square feet. Additional equipment for this research will include microtome, a cryotome to be purchased with other funds in September, 2006, and two fluorescent microscopes.
The Geosciences department maintains a small Geochemistry laboratory that has two new hoods (2001), and wet chemistry equipment suitable for conducting soil mineral analyses and determinations. The department maintains a Rigaku MiniFlex X-Ray Diffractometer running Jade 6.0 Peak Search and Match software running the Powder Diffraction File 2.0 (PDF-2) database.
The Computer Science department maintains file servers, computer servers, web servers, database servers, and three modest 16 node Beowulf clusters which are used for the development and testing of computational science curriculum modules, the teaching of parallel and distributed programming, and student/faculty research projects (see http://cluster.earlham.edu). This infrastructure is managed by a student/faculty applied computer science group, the System and Network Administrators (see http://cs.earlham.edu). The Hardware Interfacing Project, another student/faculty applied computer science group, has designed and developed a variety of field-deployable computing equipment. This includes stand-alone weather and energy monitoring systems. Computing Services at Earlham College maintains multiple Internet links for college-wide use.
10) Equipment requests should:
- Describe comparable equipment already at the institution and explain why it cannot be used.
- Explain if the new equipment will be available to support other efforts and how time will be allocated on it.
- Describe plans for facility operations and maintenance.
Large freeze dryer – There is currently one small freeze-dryer that would be available for this project. Given the large number of soil, plant, and biological samples that will be processed for this project, there is a need for a high capacity freeze dryer. The model we have requested will include bulk drying trays and a large capacity.
Acid digestion system - There is currently one microwave digestion system. Digestion of solid samples will likely be one of the bottlenecks in the analysis of samples by our atomic spectroscopy equipment. The instrument that we propose to purchase will be a CEM MARSxpress system with a high throughput capacity (40 sample tray).
Atomic Absorption Spectrometer (AAnalyst 800) and Spectroscopy supplies. Recently we have purchased an AAnalyst 800 combination graphite furnace and flame atomic absorption spectromter. We will need to purchase additional lamps and other supplies to utilize this instrument for the wide array of metals that we propose to analyze.
Semi-automated Total Organic Content Analyzer - for the more sophistocated fate and transport models as well as the biotic ligand model, it is imperative to know the dissolved organic content. We do not currently have this capability.
Differential GPS - In order to resolve distribution trends of spatially heterogeneous data, it is crucial to be able to quickly and accurately locate all environmental sampling events from every type of media. The Geosciences Department currently maintains one Trimble GeoExplorer III with post-processing Differential Global Positioning System (DGPS) capability. Our Trimble GeoExplorer III is 6 years old and is beginning to wear out. A new DGPS, dedicated to the project would speed data recovery and transfer.
Field monitoring equipment (Temperature, pH (digital), conductivity, redox (reduction oxidation potential), pressure transducer, nitrate selective probe, single board computer, packaging, and communications) - this equipment will be purchased, integrated and deployed at our field test site to provide a continuous monitoring of water quality parameters.
Field Sampling Kits (Lake sediment cores to 2 m, Shelby soil cores, Monitoring wells (one time install), Drawing equipment)
Biology sampling gear (Nets (ten 50’ fyke nets @ $300 each; Nichols Net and Twine), Containers: Rubbermaid fiberclass stackable tubs (10 @ $30), Passive Integrated Transponder (PIT) tags (250 @$7) and reader (InfoPet))- this equipment will be utilized for the population ecology studies and for the metal analysis studies
11) Plans for this project beyond the proposed time period, including financial support.
The curriculum modules developed will continue to be utilized after the grant period expires. In addition, these modules will serve as templates for the development of additional environmental modules. Although we will hold the initial workshop for faculty at other liberal arts institutions during the grant period, we would offer this workshop again if there was sufficient demand.
To continue the summer research program, we are embarking on a capital campaign that includes a goal of building a $3 million endowment for faculty/student research. The income from this endowment, along with operating funds, would provide research resources after the grant period ends. We also believe that a WMKF investment will serve as a catalyst for major gifts from alumni, friends, corporations and other foundations as we continue and expand the program.
12) Describe how the success of the project will be evaluated in terms of the goals proposed. Include information regarding outside review committees, if appropriate.
- Evaluation Team: We have identified several possible sources of both internal and external evaluation. Internal evaluations will be conducted by one or more members of the project and other faculty and staff within the science division who are not directly involved in developing or using these curricular modules. External evaluators will be drawn from program assessors who have worked previously with Earlham College and/or from organizations which provide consultancy services such as the Council on Undergraduate Research (CUR) or the W.M. Keck - Project Kaleidoscope Consultation Program (Keck-PKAL). CUR can provide program evaluation sensitive to issues at predominantly undergraduate institutions including evaluators with experience in evaluating interdisciplinary and interdepartmental programs. Keck-PKAL has strengths in program evaluation that focuses on creative approaches to the education of the STEM student population.
- Timing: Evaluation of the Keck program will occur both during (formative) and at the end (summative) of the grant period. The formative process evaluations conducted at various points throughout the grant period, particularly during year 2, and will assist the faculty and academic departments involved in this interdisciplinary project to refine the project goals and objectives and to make any ongoing modifications or revisions. They will identify what is working during the initial implementation and point to areas needing further development. The summative evaluation will take place at the end of the grant period and will provide a measure of how well the program goals were met as well as provide directions for future growth.
- Goals: The project leaders will meet with the evaluators to clarify, operationalize, and select and develop instruments which evaluate the stated and implicit goals of the project (above). The project evaluators will also help to conceptualize key issues or problems that would keep our program from meeting our stated objectives and specify particular criteria for success as well as identify particular data needed to determine how well the components of the program are meeting their objectives.
- Methods: Delineating project goals will assist us in developing both qualitative and quantitative measures for determining how well our goals are being met during both the formative and summative evaluation phase. Qualitative evaluations will include:
- Open-ended surveys: The evaluator will collect answers without preset response categories to written questions. Surveys with an open-ended component would be given to all students enrolled in courses where a research-based interdisciplinary module was used, faculty who developed and taught such courses, and participants in any workshops in which the faculty involved in the Keck program presented the pedagogy and organization of this project.
- Semi-structured interviews: The evaluator will conduct semi-structured interviews of key personnel and a representative sample of students to allow the evaluator some first hand experience with the evaluated activities and a chance for in-depth exploration of particular issues.
- Peer evaluation: Efforts at developing interdisciplinary course modules incorporating computational modeling will be described in peer-reviewed publications providing feedback from the reviewers as well as others who read the articles.
Quantitative evaluations will include:
- Quantitative surveys: Quantitative pre and post surveys of student or workshop participant attitudes toward and confidence in computational methodology, interdisciplinary collaboration, relevance of environmental studies in particular disciplines, and interest in science and society.
- Institutional data: Assessment of pre and post grant levels of student participation in undergraduate research, likelihood of taking a second science class, and number of science majors.
- Incorporation in curriculum: Measurement of the pre and post grant percentages of incorporation of computational modeling in primarily field or laboratory based courses and field or laboratory modeling in computational courses.
- Incorporation in co-curriculum: Comparison of the numbers pre and post grant interdisciplinary projects including collaborative research projects and grant writing efforts.
- Results: Final reports summarizing both the quantitative and qualitative data will be produced. They will assess which and to what degree goals have been met for affected students, faculty, and the institution as a whole as well as provide recommendations for further implantation and dissemination.
Dissemination activities will include:
- NITLE workshop on integrating multi-disciplinary computational methods into the undergraduate science curriculum.
- Earlham Science Poster Session (held each Fall)
- Student presentation of papers at regional and national scientific conferences (Butler Undergraduate Research Conference, Geological Society of America, American Chemical Society, etc).
- CUR publications and programs
- Student/Faculty papers in science pedagogy journals and basic science journals, as appropriate
4) Project Budget Form
- Do we have a spreadsheet template to work from?
- Upload spreadsheet to <here>.
5) Budget Narrative
1. Provide a brief justification of each budget line item.
Personnel – Salary & Fringe Benefits (Total 3 year budget $ 227,613 : $201,777 grant funded, $25,836 institutional funding) 48 Faculty weeks per year(total 144 faculty weeks) @ $600 per week + .0765 FICA and Medicare for summer research and curricula work (e.g. 6 faculty x 8 weeks per year) 96 student weeks per year (total 288 student weeks) @ $400 per week +.0765 FICA and Medicare for collaboration with faculty in research and curricula work (e.g. 12 students x 8 weeks per year) Grant support @ $3,000 + .0765 FICA and Medicare + 10% TIAA/CREF as staff for, reporting, purchasing supplies, budget monitoring, etc. Equipment (Total 3 year budget $100,000 - $80,800 grant funded, $19,200 institutional funding) Large freezer dryer @ $15,000 – drying of biological and soil samples in preparation for digestion and analysis.
Acid digestion system @ $17,500 – dissolution of biological and soil samples prior to analysis by GFAAS or ICP-AES
Atomic Absorption Spectrometer – college funded ($19,200) – analysis of low levels of toxic metals such as lead and arsenic
Semi-automated Total Organic Content Analyzer @ $12,000 - for the more sophisticated fate and transport models as well as the biotic ligand model, it is imperative to know the dissolved organic content. We do not currently have this capability.
DGPS @$2250 – will be used to precisely locate sampling sites in our remote sampling location (Springwood Lake)
Field monitoring equipment – 4 sets @ $3,000 will include electronic and computer components necessary for the construction of field monitoring stations that will be used to continuously monitor temperature, pH (digital), conductivity, redox potential, pressure, and nitrate levels at our on-campus field site.
Field sampling equipment @ $15,000 – will be used to construct our on-campus and remote field sites including construction of a monitoring well and equipment for obtaining sediment and soil cores.
Biology sampling gear @ $3,800 – will include equipment such as nets and electronic identification tags that will be used to capture and/or monitor biological samples such as fish and turtles.
Operations (Total 3 year budget $198,313 - $76,300 grant funded, $122,013 institutional funding
Consumable supplies @ $25,333 per year - will include lab supplies necessary for sample processing and atomic analysis for both academic year and summer, which will impact X students per year (e.g. clean acids, consumables for atomic spectroscopy (graphite tubes, Ar gas), pipet tips)
Travel/symposiums @ $11,100 per year – will fund faculty and student travel to regional and national meetings to present the results of this research as well as the costs of poster.
Facilities Overhead – All institutional funded.
Evaluation – @ $3000 per year – construction and analysis of surveys........
Library Acquisitions @ $2000 per year – will fund purchase of relevant texts as well as interlibrary loans.
2. State the number of students (undergrad/grad/postdoc), research associates or technicians to be supported and number of years of support.
The project will support summer research for approximately 12 students each summer for 3 years. The project also will impact nearly every one of the 1200 students enrolled at the College who will take one or more of the courses designed for this project.
3. Explain why W. M. Keck Foundation support is essential for this project.
Funding for undergraduate research at small, liberal arts colleges is limited. The WMKF is known and respected throughout the scientific community as a foundation that supports innovative science programs at high-quality undergraduate institutions. WMKF funding would allow Earlham to launch its multidisciplinary science project. Keck support would also raise the visibility of the sciences within the College and with external audiences regionally and nationally.
Earlham College will provide $167,000 specifically toward this project. There is no other pending funding. However, with the leverage and prestige that a Keck grant will provide, we believe that we will be well-positioned to solicit major gifts (both for current use and for an endowed student/faculty research fund) for continuation and expansion of multidisciplinary science projects, as well as submission of proposals for future funding to NSF and other private foundations.
5. If construction or remodeling is involved, provide a copy of the permits required or an explanation of how and when the permits will be acquired.
There are no construction or remodeling components of this proposal.
6) Recognition Statement
- Describe the manner in which the institution will recognize and acknowledge a W. M. Keck Foundation grant for this project.
Earlham College science faculty and public information staff will work closely with the W.M. Keck Foundation to publicize grant funding. Upon approval of text, Earlham's public information staff will distribute news releases to local, regional and national media, and to educational publications such as the Chronicle on Higher Education to announce receipt of a Keck grant.
NITLE workshop paragraph, note letter of support.
CUR paragraph.
In addition, a feature article for the Spring 2007 Earlhamite, Earlham's alumni magazine, will be prepared and submitted to the Foundation for approval. The Keck award will be highlighted on the Earlham College web page with links to news coverage and the Earlhamite article. Keck will also be acknowledged on our Natural Sciences web page (http://www.earlham.edu/naturalsciences/), and on each participating discipline web page.
Faculty and student written and oral presentations on the multidisciplinary curriculum development and/or research projects will acknowledge Keck support. These presentations could include journal articles, and papers and poster sessions at regional and national conferences, as well as in publications in organizations such as the Council for Undergraduate Research (CUR).
7) Project Documents
- 1. Biographical sketches (limit 2 pages for each investigator)
- Required for key personnel at the applicant institution and collaborators at other institutions.
- Each sketch should contain name, position title, organization, contact information included e-mail address, degrees, years and field of study for each academic degree, a listing of research and professional positions, awards, and honors, and references to all publications for the past three years along with any earlier publications pertinent to this application.
- 2. Collaborative Arrangements: Provide letters from the collaborators and the director of their institution(s) endorsing this request and the role of each collaborator. Please include contact information including e-mail addresses.
- We already have some of this in the phase I submittal.
- NITLE letter - Nancy Millichap (or the director); support for logistics, advertising, and hosting of a dissemination workshop.
- City of Richmond/Parks Department
- IDEM
- Letters should be sent to us for inclusion and addressed to:
Mat Varma, Ph.D. Program Director W. M. Keck Foundation 550 S. Hope Street, Suite 2500 Los Angeles, CA 90071
- 3. Bibliography of the literature cited in the project.
- Make sure we have sited everything we list? Is it that formal of a document?
- bibliography